2025.07.17

MGC’s OXYCAPT™ Receives Packaging Innovation Award at Pharmapack 2025, Europe’s Largest Medical Packaging Event

Mitsubishi Gas Chemical Company, Inc. (MGC) recently received a prestigious medical container industry award for the first time. But how was the globally unique OXYCAPT™ product developed and why was it recognized?
In January 2025, MGC received its first ever Packaging Innovation Award at Pharmapack 2025, held in Paris by Informa Markets. It won the award for its OXYCAPT™ product.
Pharmapack, established in 1997, is Europe’s largest event for pharmaceutical packaging and medical devices. Attracting experts and industry professionals from 75 countries worldwide, Pharmapack has maintained a leading position in Europe since its inception. MGC has participated in this event as an exhibitor since 2012, and this year won the innovation award with its first-ever entry. The product has already attracted numerous inquiries from experts and professionals across the industry.
OXYCAPT™, which received the award, is a three-layer container that combines the advantages of both glass and plastic. The inner and outer layers are made of cyclo olefin polymer (COP), a well-established material in the pharmaceutical industry, while the middle layer contains a gas barrier polymer developed by MGC. The product delivers excellent barrier properties against O2, CO2, and UV, as well as low inorganic extractables, low protein adsorption, and outstanding break resistance at cryogenic temperatures. Its O2 and CO2 permeability is about one-twentieth that of COP monolayer containers. Despite its transparency, OXYCAPT™ also offers strong UV barrier performance. These excellent properties contribute to the long-term storage of drugs that are sensitive to O2, CO2, and UV.
“OXYCAPT™ is a proprietary product developed solely by our company,” enthuses Tomohiro Suzuki, associate general manager of the Health Technology & Solution Department in the Research & Development Division. “At present, it is a unique solution unmatched anywhere in the world, with no competing products.”
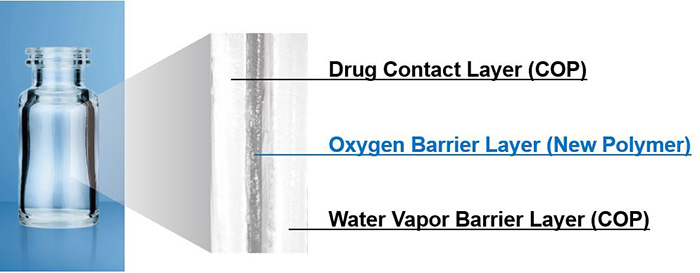
OXYCAPT™ ensures long-term stability of cell and gene therapy products
with superior gas barrier performance
Traditionally, medical containers such as syringes and vials have primarily been made of glass. However, with the recent advancement of new modalities such as cell and gene therapy products, containers are increasingly required to withstand deep freezing and transportation with dry ice, specifications that occasionally render conventional glass and plastic containers unsuitable. OXYCAPT™, with its excellent gas barrier properties, demonstrates outstanding performance even under these kinds of harsh conditions, contributing to the long-term stability of cell and gene therapy products. This was a key reason for the presentation of the award. Tomoya Nakatani from the Health Technology & Solution Department in the Research & Development Division shares his thoughts on receiving the award:
“Going to Paris for this was my first overseas business trip, and I was truly honored to receive our first-ever award there, and at such a grand and deeply moving ceremony. The product is the fruit of my predecessors’ dedicated efforts. Through this experience, I learned a great deal about passion and having the right attitude at work.”
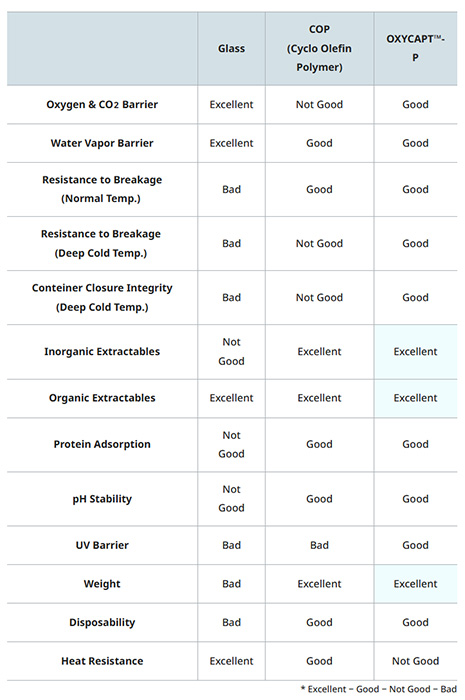
Advantages of OXYCAPT™
Suzuki also shared his feelings and experience about the event, saying, “I have been involved in this project for over ten years. Receiving this award truly feels like a reward for all the hard work we’ve put in. Going forward, I hope to contribute to the medical field by supporting drug development from the early stages with this one-of-a-kind product.”

At the award ceremony
Hard work over time will eventually pay off.
What truly matters is not giving up.
OXYCAPT™, whose development began more than a decade ago, has been sold on a trial basis by the Research & Development Division for the past several years.
“MGC had already accumulated knowledge and expertise in manufacturing three-layer bottles, using its product MX-Nylon in the middle layer of beverage bottles,” Suzuki explains. “The idea of applying this technology to a different field led to the development of a medical container. MGC presented the concept at Pharmapack even before the product was complete, actively promoting it to the industry.”
This is MGC’s first initiative of this nature. It was undertaken as part of the Next Generation Business Project launched more than a decade ago, in which the development of medical containers was selected as a key theme.
“Normally, we develop new products as extensions of existing ones,” says Suzuki. “But this time, we ventured into entirely uncharted territory—a true frontier field. Moreover, the medical industry is extremely cautious by nature when adopting new technologies. This makes sense, as any product defect could lead to fatal consequences. That’s why we began with limited trial sales to build a track record. The product was developed gradually, through trial and error over an extended period, by a small and dedicated team.”
With a strong emphasis on research and development, MGC has nurtured many products through a long-term approach that sets it apart from other companies. Looking ahead, it aims to establish OXYCAPT™ as the de facto standard for use in biopharmaceuticals, as well as in gene and cell therapy products that are highly sensitive to O2 and CO2. The company also plans to accelerate its marketing efforts by exhibiting the product at trade shows and conferences, contributing more articles to industry journals, and expanding adoption of OXYCAPT™ among pharmaceutical companies in Japan, the U.S., and Europe that are developing cutting-edge modalities.
“Hard work over time will eventually pay off,” Suzuki concludes. “A key factor behind the success of this research was the discovery of potential applications beyond what we had originally envisioned. Recognition of this unexpected value enabled us to accumulate data. The initial breakthrough was purely coincidental. What truly matters is not giving up. Research and development involves trial and error, along with the flexibility to adapt. I believe this mindset is one of MGC’s greatest strengths.”
INTERVIEWEES

TOMOHIRO SUZUKI
Associate General Manager
Health Technology & Solution Department, Research & Development Division

TOMOYA NAKATANI
Health Technology & Solution Department, Research & Development Division
Mitsubishi Gas Chemical Company, Inc.
Mitsubishi Building, 5-2 Marunouchi 2-chome, Chiyoda-ku, Tokyo
Mitsubishi Gas Chemical Company, Inc. (MGC) traces its origins to Edogawa Barium Industry Co., Ltd., which was established in 1918 through an investment by Mitsubishi Paper Mills Limited. The company was renamed Mitsubishi Edogawa Chemical Co., Ltd. in 1962. In 1971, it merged on equal terms with Japan Gas Chemical Co., Inc., founded in 1951, to form Mitsubishi Gas Chemical Company, Inc. The company updated its official Japanese name in 1991, while retaining its English name. As of the end of March 2024, the company has 7,918 consolidated employees. MGC is the world’s only fully integrated methanol manufacturer and holds the top global market share in products such as optical resin polymers.
